- Index
- >Projects
- >Projects in progress
- >RECOME
RECOME Research Project
Reliability of Electronic COmponents for MEdical devices
Group : Reliability Engineering and Decision-Making tools
Labelling: none
Duration: 36 months (from April 2021 to November 2025)
Funding: Pays de la Loire Region (for LARIS) and SATT Bretagne Pays de Loire
Staff involved from LARIS: Abdessamad KOBI, Laurent SAINTIS, Mihaela BARREAU, Bruno CASTANIER, Fabrice GUERIN, Fatima-Ezahra INDMESKINE (IGR)
Project partners: Tronico company and SATT Bretagne Pays de Loire
Official website : https://www.recome.org/
Abstract:
 Electronics are increasingly present in implanted medical equipment. Until now, their reliability has had little impact, but more and more frequently a failure of the electronics has an immediate impact on the patient, whether through the need for emergency surgery (artificial sphincters) or the death of the patient (artificial heart). Moreover, the estimation and demonstration of reliability is essential for the authorisation of the equipment to be placed on the market.
Electronics are increasingly present in implanted medical equipment. Until now, their reliability has had little impact, but more and more frequently a failure of the electronics has an immediate impact on the patient, whether through the need for emergency surgery (artificial sphincters) or the death of the patient (artificial heart). Moreover, the estimation and demonstration of reliability is essential for the authorisation of the equipment to be placed on the market.
The control of reliability is not new in electronic systems. Its massive use in aeronautical equipment over the years has shown that its use does not increase accidents, quite the contrary. However, for aeronautical equipment, reliability is managed at the electronic system level by "redundancy, dissimilarity". The equipment is duplicated (redundancy), the duplicates being designed by different teams, with different development tools and incorporating different electronic components (dissimilarity). In this way, the risk of all the electronic equipment necessary for the operation of a system failing simultaneously is almost zero (less than one failure per hundred years of flight of the entire world fleet).
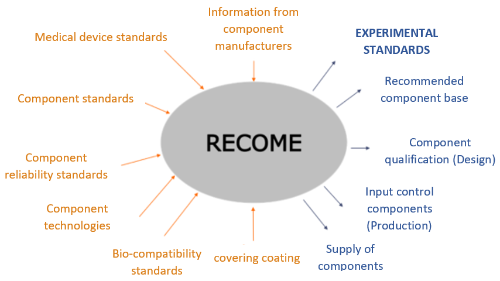
For implanted medical equipment, full redundancy is not possible due to the limited volume of implants. Therefore, the reliability of the system depends directly on the reliability of the electronic components themselves. To date, there is no "medical grade" for electronic components. Some component manufacturers offer this grade in their catalogue, but its meaning from one manufacturer to another varies greatly. The aim of the RECOME project is to create an environment for demonstrating the reliability of components for medical use:
- Optimisation of reliability models
- Validation of reliability models
- Drafting of an experimental standard
- Creation of a database of qualified component batches
- Distribution of qualified component batches
To do this, we will have the following input data, which we will have to optimise, validate and complete through our research operations:
- The information from the component manufacturers rarely mentions implemented uses. Their reliability in this specific environment will have to be assessed
- Standards and regulations for medical devices give targets for the overall system that will have to be achieved in relation to the targets we will define at component level
- Component standards give indications on their ageing which will have to be adapted to the specific environment and validated
- Component reliability guidelines (e.g. FIDES) will need to be verified for sub-miniature components in the specific environment
- Component technologies are the main drivers of reliability, yet they are often confidential and will need to be established to determine their ageing
- Bio-compatibility standards impose additional stresses on components not considered in reliability models (sterilisation, specific coating...)
It is envisaged that this work will be carried out on 10 major families of components, each represented by 5 component references in order to be representative of the manufacturers present on the market.


