- index
- >Projets
- >Projets antérieurs
- >IRM et AVC de l'enfant
Projet de recherche
“Analyses des données d'imagerie IRM et AVC de l'enfant et modèle de croissance physique”
Équipe : Information, Signal, Image et Sciences du Vivant
Labellisation : aucune
Durée : 3 ans (2018 - 2021)
Financement :
Personnels impliqués du LARIS : Mickael Dinomais (Chef de service de Médecine Physique (CHU-Angers)), Mariam Al Harrach (post-doctorante)
Partenaires du projet : François Rousseau (Professor, expertise in perinatal image processing methods, Brest), Julien Lefèvre (Associate professor, expertise in neonatal brain morphometry, Marseille)
With a prevalence of 1/2,000 to 1/4,000 live births, perinatal ischemic stroke is the most frequent form of childhood stroke and constitutes the leading cause of unilateral cerebral palsy in term-born children. Perinatal ischemic stroke is an umbrella term including several conditions that differ in pathophysiology, timing and thus in outcomes. Neonatal arterial ischemic stroke (NAIS) refers to a perinatal ischemic stroke syndrome with neonatal signs (mainly iterative focal seizures in the first days of life) related to an arterial infarct as revealed by brain imaging.
Every case of NAIS is unique to the individual. Considering for instance NAIS leading to unilateral cerebral palsy, one child may have total motor deficit and require early intensive motor rehabilitation therapy, while another with no or mild motor impairment require no or less motor rehabilitation approach. This decision of proposing or not such motor therapy impact the family life, and the decision must be taken in awareness of necessity of such therapy. The variability of the motor prognosis after early brain lesion as represented by NAIS is due in part by the localisation, type of injury and the timing of the injury to the developing brain. The prediction of long-term motor outcome requires new personalized approaches, i.e. patient-specific techniques to understand the causes of the observed disabilities.
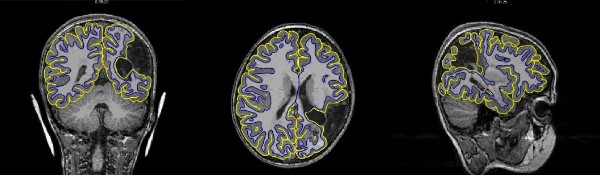
Figure1: Depiction of gray and white matter boundary estimation on T1-weighted image of a 7 year old after NAIS
Magnetic Resonance Imaging (MRI) is an extremely powerful imaging modality for in vivo human brain studies. The use of MRI led to recent breakthroughs for biomarker discovery during early brain development, related to the potential correlations between specific tissue lesions, volumetric variations of given cerebral structures, or brain folding, on the one hand, and some disabilities, mainly motor, on the other hand. Recent works describe also how computational modeling of brain growth starts to bridge the scales (from the cellular level toward form and function at the brain level) to make efficient predictions. Physics-based models are a cornerstone in such studies, by quantifying for instance cortical stresses and predicting gyrification indices.
On the one hand, physics-based approaches provide new realistic brain growth models enabling the simulation of major biological processes such as cortical folding occurring during early development (see our recent work in Tallinen et al. 2016). On the other hand, image-based approaches provide new observation techniques for in vivo quantification in 3D and 4D of the growing brain (Rousseau et al. 2016, Lefèvre et al. 2016).
The main objective of this project is to improve predictive power of the impact of NAIS on brain growth and motor disabilities in order to propose well suited therapy. To reach this goal, the project consists in developing an enhanced physically-based brain growth numerical model by including accurate brain morphometry and brain network geometry (i.e. so called connectome) extracted from MRI. This multidisciplinary project aims at providing new biomarkers dedicated to NAIS exploiting the joint use of physics-based growth models and medical image analysis.
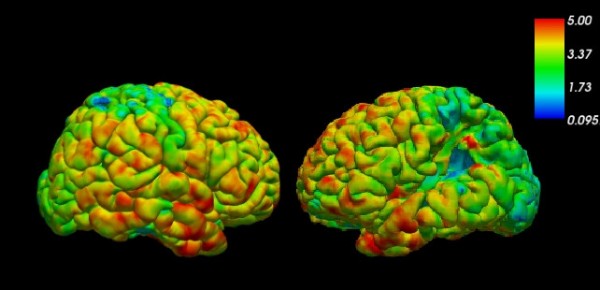
Figure 2: Cortical thickness estimated with Freesurfer


